Introduction to DMF and DMAc
DMF and DMAc are high-boiling, polar aprotic solvents with excellent solvating power. They are workhorses in industries like:
- Polymer Industry: Production of polyurethane, polyacrylonitrile (acrylic fibers), and PVC.
- Pharmaceutical Industry: As a reaction medium for APIs.
- Agrochemicals: Synthesis of pesticides and herbicides.
- Electronics: In the manufacture of printed circuit boards and lithium-ion batteries.
Because they are expensive and have significant environmental and health impacts, their recovery and reuse are not just economically advantageous but often a regulatory necessity.
The Recovery Process: An Overview
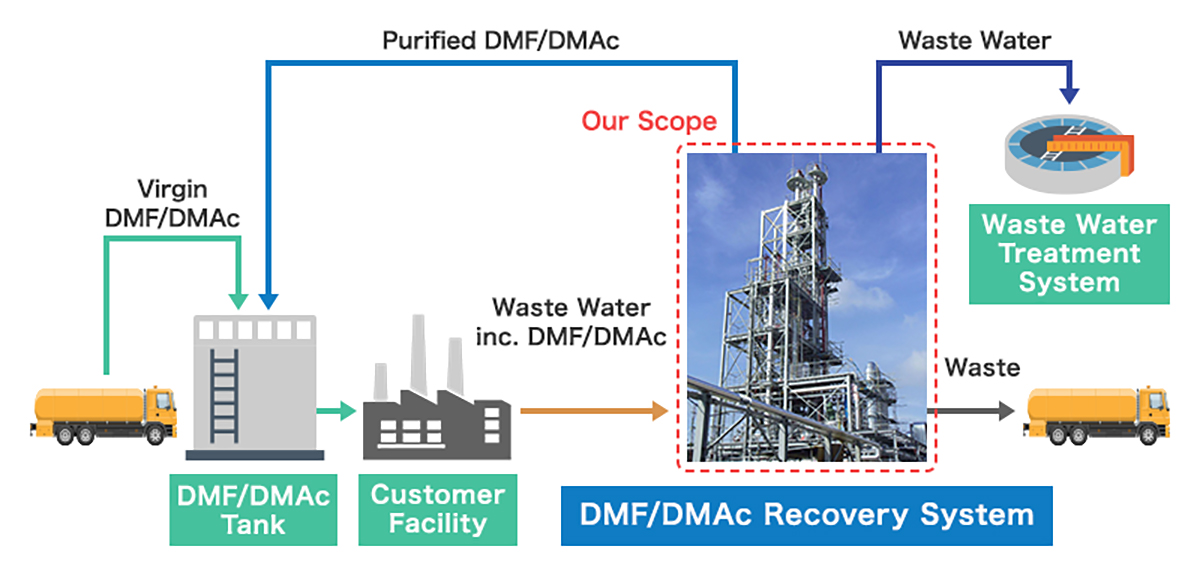
The recovery process aims to separate pure DMF or DMAc from a waste stream, which is typically a mixture of the solvent, water, and various organic or inorganic impurities. The most common and efficient method is Distillation, often enhanced with specific techniques to overcome challenges like high boiling points and azeotrope formation with water.
The general process flow can be summarized as follows:
Key Steps in Detail
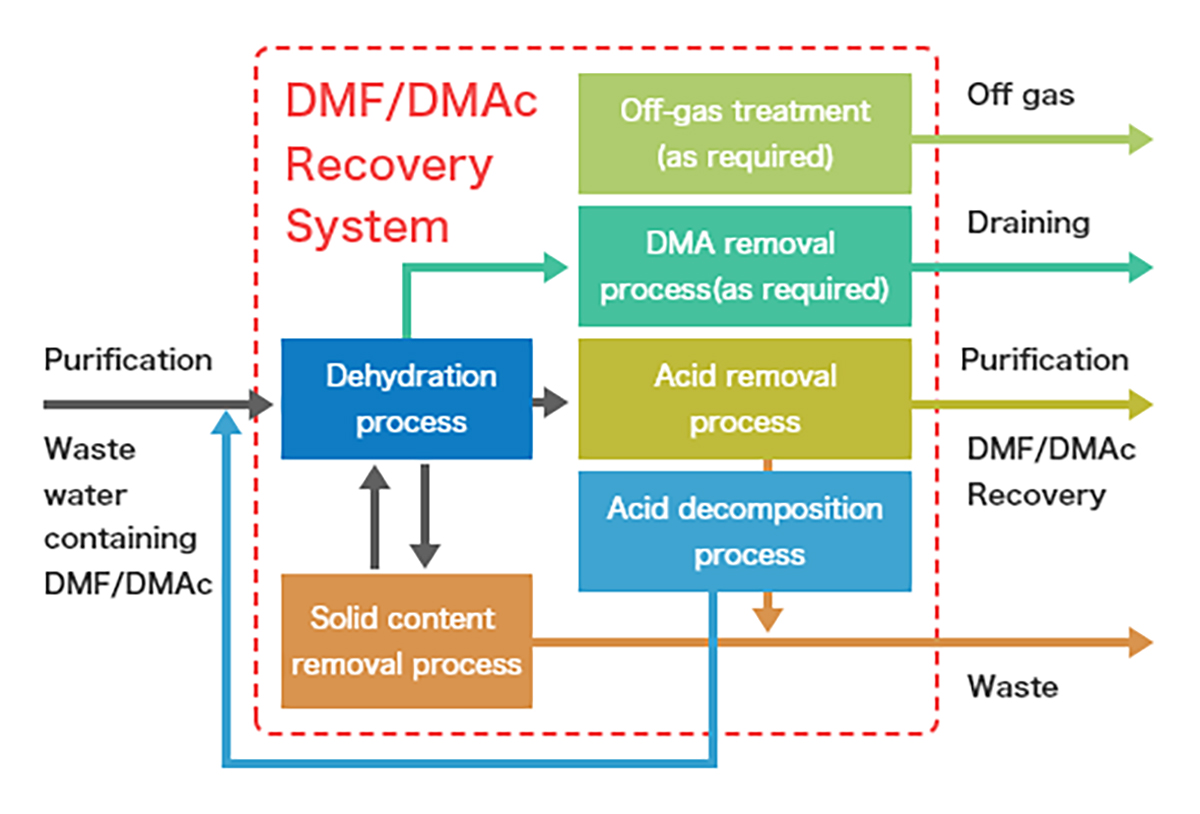
1. Pre-Treatment
Before distillation, the waste solvent often requires preparation.
- Filtration: Removal of suspended solids, polymers, or catalysts.
- Settling/Decanting: If water is present in large quantities and immiscible, it can be partially separated by gravity.
2. Primary Distillation (Solvent/Water Separation)
This is the core of the recovery process. The challenge is that both DMF and DMAc form azeotropes with water.
- DMF-Water Azeotrope: Boils at ~99.5°C and contains ~36% water.
- DMAc-Water Azeotrope: Boils at ~93°C and contains ~23% water.
This means a simple distillation will not yield pure, dry solvent. Two main approaches are used:
a) Low-Pressure (Vacuum) Evaporation / Distillation
- This is the most common industrial method.
- Applying a vacuum lowers the boiling point significantly, saving energy and preventing thermal decomposition of the solvent or any heat-sensitive impurities.
- The distillation column is designed to break the azeotrope. The overhead distillate is the near-azeotropic mixture of water and solvent, leaving a concentrated, wet solvent at the bottom.
- The water-rich overheads are condensed, and the two phases (a water phase and a solvent phase) can often be separated in a decanter. The solvent-rich phase is refluxed or fed back into the system.
b) Extractive Distillation
- In this method, a high-boiling, non-volatile “entrainer” (like glycerol or ethylene glycol) is added to the column.
- This entrainer alters the volatility of the components, breaking the azeotrope and allowing pure water to be taken overhead while the mixture of DMF/DMAc and the entrainer is collected at the bottom.
- A second distillation step is then required to separate the solvent from the entrainer, which is recycled.
3. Fine Distillation & Drying
The concentrated solvent from the primary column still contains some water and light/heavy impurities.
- Drying: To remove the final traces of water, methods like:Fractionation: The dried solvent may undergo a final precision distillation to separate light-end impurities (like methylamines or acetic acid from DMAc decomposition) and heavy-end impurities (like dyes, salts, or polymers). This step produces a high-purity recovered solvent.
- Vacuum Distillation: Under high vacuum, the remaining water can be driven off.
- Molecular Sieves: Passing the solvent through beds of 3Å or 4Å molecular sieves is a highly effective way to adsorb residual water.
4. Post-Treatment of Water Stream
The water stream from the primary distillation, while mostly pure, will contain ppm levels of solvent. This water must be treated before discharge to meet environmental regulations.
- Activated Carbon Absorption: The most common method, effectively removing trace organic solvents.
- Biological Treatment: In an integrated wastewater treatment plant.
- Advanced Oxidation Processes (AOPs): For stubborn contamination.
Process Considerations & Challenges
- Energy Consumption: Distillation is energy-intensive. Heat integration (using the vapor from one column to reboil another) and multi-effect evaporators are crucial for economic viability.
- Thermal Stability: DMF and DMAc can decompose at high temperatures, especially in the presence of acids or bases. DMF can decompose to dimethylamine and carbon monoxide. DMAc can decompose to dimethylamine and acetic acid. Operating under vacuum is key to minimizing this risk.
- Corrosion: The decomposition products (e.g., acetic acid from DMAc) are corrosive. The presence of water and salts can exacerbate this. Construction materials like stainless steel (SS316L) are often required.
- Purity Requirements: The required purity of the recovered solvent depends on its reuse application. Pharmaceutical use demands much higher purity than, for example, a polymer spinning bath.
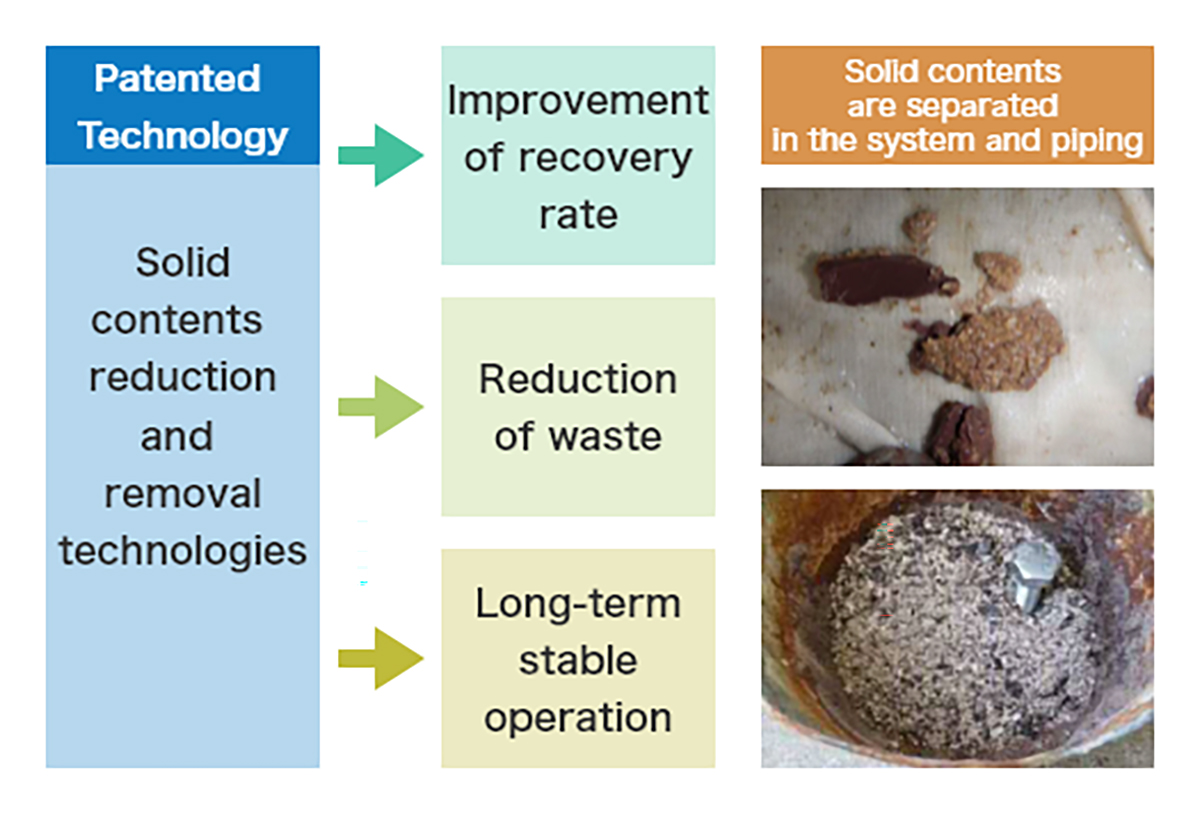
- Residue Handling: The bottom residue from the distillation (still bottoms) contains concentrated impurities, salts, polymers, and tars. This hazardous waste must be disposed of or incinerated properly.
Comparison of DMF vs. DMAc Recovery
|
Feature |
DMF Recovery |
DMAc Recovery |
|
Azeotrope with Water |
~99.5°C, ~36% water |
~93°C, ~23% water |
|
Decomposition Products |
Dimethylamine (DMA), CO |
Dimethylamine (DMA), Acetic Acid |
|
Corrosivity |
Less corrosive, but CO is a safety hazard. |
More corrosive due to acetic acid. |
|
Drying |
Slightly easier due to lower water solubility. |
Requires careful drying to remove acetic acid traces. |
|
General Difficulty |
Slightly less challenging. |
Can be more challenging due to corrosion and acidity. |
Conclusion
The recovery of DMF and DMAc is a mature but critical industrial process. It is a balance of chemistry and chemical engineering, primarily relying on low-pressure distillation to efficiently separate the solvent from water and impurities while minimizing decomposition. A well-designed recovery system can achieve recovery rates of over 99.5%, leading to massive cost savings, reduced environmental footprint, and improved process sustainability.
Post time: Oct-17-2025