Why Recover DMF?
DMF (N,N-Dimethylformamide) is a high-boiling, polar aprotic solvent with excellent solvating power, used extensively in:
- Polyurethane (PU) & Acrylic Fiber Production: The largest industrial application.
- Pharmaceutical Industry: As a reaction solvent.
- Electronics Industry: In the production of printed circuit boards and semiconductors.
- Adhesives, Paints, and Coatings.
Recovering DMF is not just beneficial but often economically and environmentally mandatory because:
- High Cost: Virgin DMF is expensive. Recovery significantly reduces raw material costs.
- Environmental Regulations: DMF is classified as a substance of concern. Discharging it into the environment or incinerating it is heavily regulated and costly.
- Sustainability: Recovery aligns with circular economy principles, reducing waste and the carbon footprint of downstream products.
- Process Efficiency: In processes like PU coagulation, DMF is mixed with water. Recovering it allows for the recycling of the water stream as well.
The Core Challenge
The most common feed for DMF recovery is a wastewater stream (e.g., from a PU coagulation bath) containing:
- ~15-30% DMF
- ~70-85% Water
- Small amounts of impurities: salts, monomers, oligomers, dyes, and other organic contaminants.
The goal is to separate the DMF from water and impurities to achieve a purity level suitable for reuse (typically >99.5%). The primary challenge is that DMF and water form a homogeneous mixture with a high boiling point and an azeotrope, making simple distillation inefficient.
Primary Recovery Technology:
Distillation
The industrial standard for DMF recovery is a multi-stage distillation process, almost always under vacuum. Operating under vacuum (reduced pressure) is crucial because:
- It significantly lowers the boiling points of both DMF (bp: 153°C at atm) and water (100°C at atm). This reduces thermal stress, saves energy, and prevents thermal decomposition of DMF and any impurities.
- It helps break the azeotrope, improving separation efficiency.
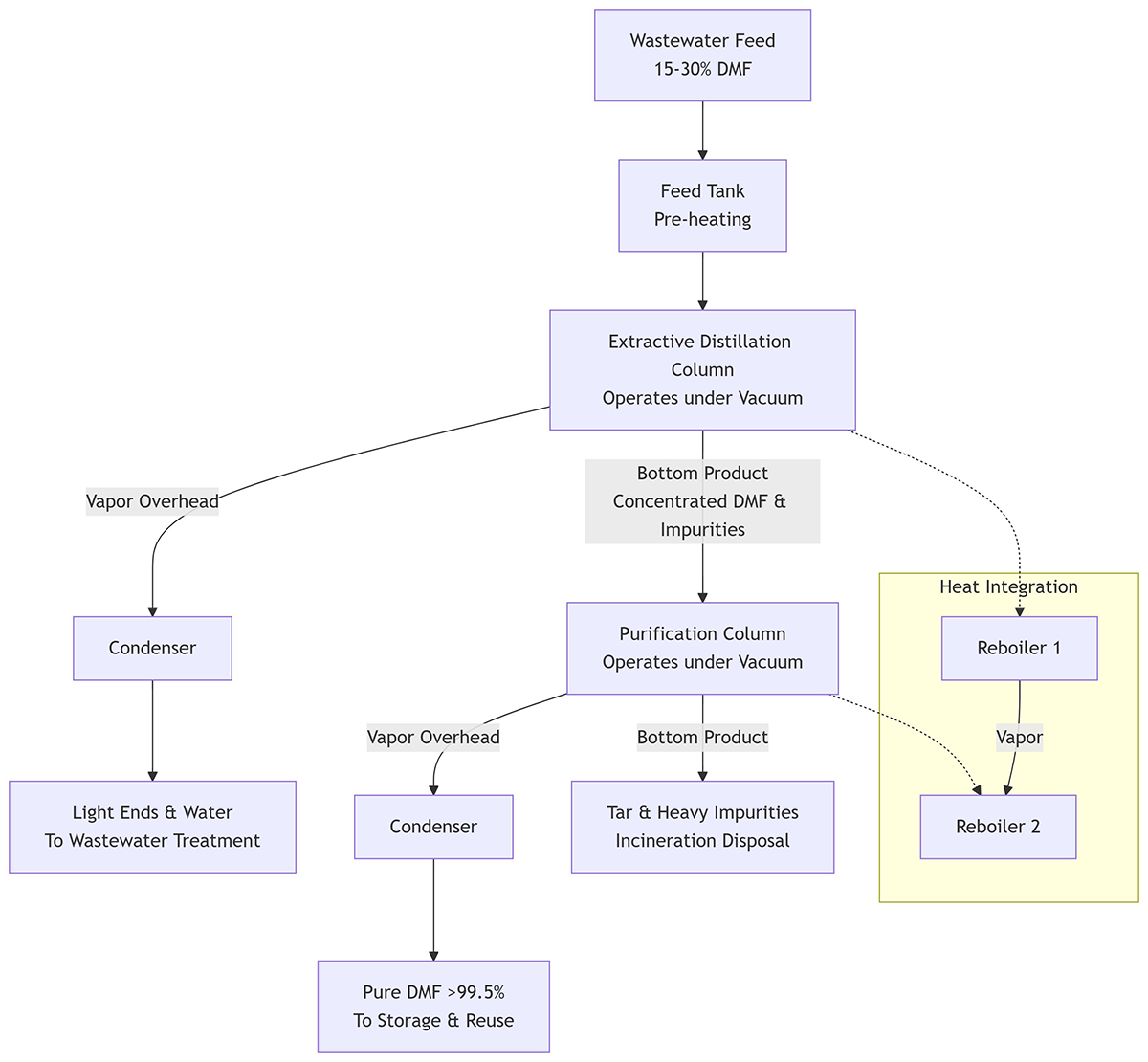
A typical system consists of two main columns:
1. Extractive Distillation Column (or Pre-Concentrator Column)
- Purpose: To remove the bulk of the water from the feed stream.
- Process: The wastewater feed is introduced near the top of the column. A high-boiling-point solvent (often DMF itself in a closed-loop system) is introduced near the top as an “entrainer” or extractive agent.
- This agent alters the volatility of the components, making water the more volatile component and allowing it to be distilled off as the overhead product.
- The bottom product, now a more concentrated DMF stream (e.g., 80-90% DMF) with the entrainer and heavier impurities, is sent to the next column.
2. Purification / Rectification Column
- Purpose: To produce high-purity DMF and separate it from heavy-end impurities (tar).
- Process: The concentrated stream from the first column is fed into the second column.
- Overhead Product: High-purity DMF vapor (>99.5%) is drawn off the top, condensed, and sent to a storage tank for reuse.
- Bottom Product: The non-volatile impurities (salts, polymers, tars) are concentrated and removed as a viscous waste stream. This “still bottoms” or “tar” must be disposed of appropriately, often by incineration.
Energy Integration: To maximize efficiency, the vapor from the first column’s reboiler is often used to heat the second column’s reboiler (using a thermosiphon or forced circulation reboiler). This cascading heat integration drastically reduces steam consumption.
Alternative and Emerging Technologies
While distillation dominates, other methods are used, especially for specific scenarios:
- Pervaporation (Membrane Separation):
- A promising technology for pre-concentrating DMF streams. A membrane selectively allows water to permeate through, leaving a concentrated DMF solution on the feed side. This can significantly reduce the energy load on the downstream distillation columns.
- Liquid-Liquid Extraction:
- Uses a solvent (e.g., chlorinated hydrocarbons) in which DMF has a high affinity. The DMF is extracted from the wastewater into the solvent phase. The DMF is then separated from the extraction solvent via distillation. This is less common due to the handling of additional solvents.
- Salting Out:
- Adding a salt (e.g., Potassium Carbonate, K₂CO₃) reduces the solubility of DMF in water, causing it to separate into a distinct phase. This can be effective for pre-concentration but introduces the challenge of handling and regenerating the salt.
Simplified Process Flow Diagram (PFD)
The following diagram illustrates the typical two-column vacuum distillation process:
Key Operational Considerations
- Corrosion: DMF decomposition at high temperatures can form acidic by-products (like formic acid and dimethylamine). Therefore, equipment is typically constructed from stainless steel (SS 316L or higher grade) to resist corrosion.
- Foaming: The presence of polymers and surfactants can cause severe foaming in the columns, which reduces efficiency and can lead to carryover. Anti-foaming agents are often used.
- Purity Monitoring: The purity of the recovered DMF is continuously monitored, often using online density meters or gas chromatographs, to ensure it meets specifications for reuse.
- Tar Handling: The disposal of the viscous bottom residue is a cost and logistical challenge. Systems must be designed to handle this material efficiently (e.g., using tar pumps, cooled screw conveyors).
In summary, DMF recovery is a mature but critical process that transforms a costly waste problem into a valuable circular resource, primarily through sophisticated, energy-integrated vacuum distillation technology.
Post time: Sep-05-2025